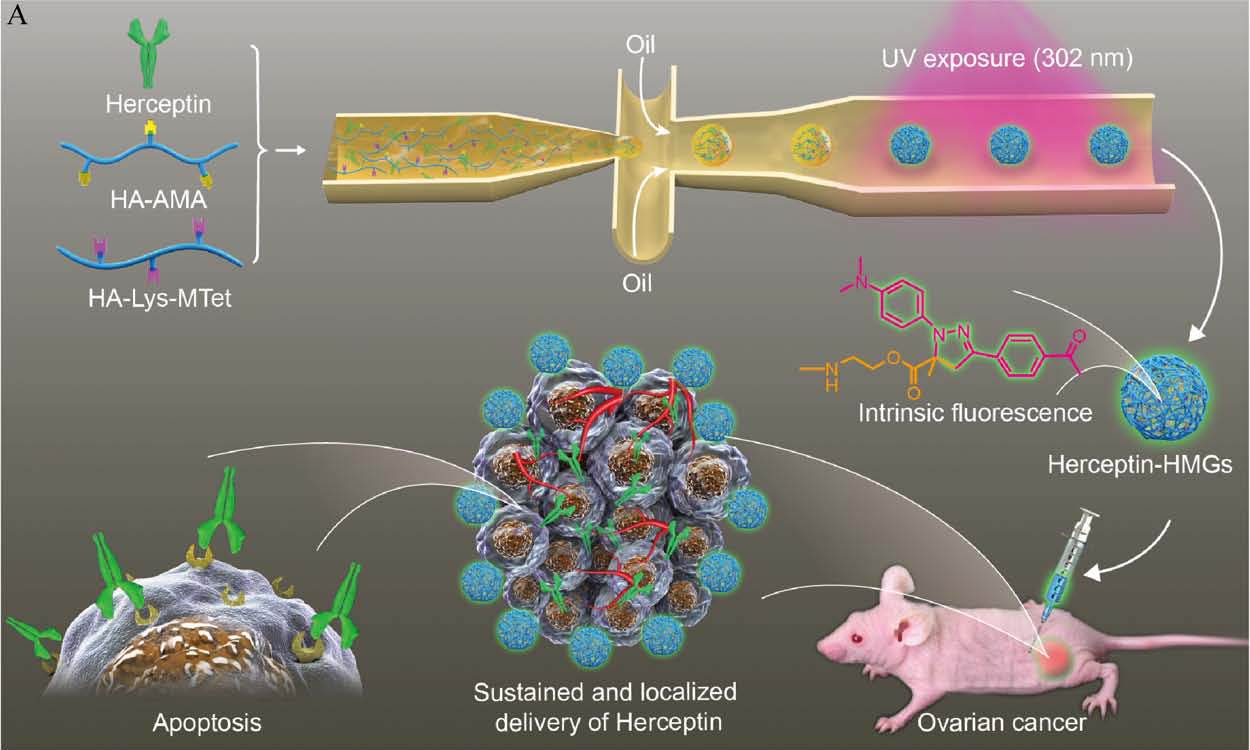
Antibody therapeutics, though representing a most used biomedicine, suffers from poor in vivo stability, rapid degradation, and frequent injections. Here, we report that fluorescent hyaluronic acid microgels (HMGs) tailor-made by combining microfluidics and “tetrazole−alkene” photoclick chemistry enable sustained and localized delivery of Herceptin in ovarian tumors. HMGs were obtained with a defined size (25−50 μm), narrow size distribution, high stability, and strong green fluorescence. Notably, HMGs exhibited a remarkably high loading of proteins such as Herceptin and IgG with a loading efficiency exceeding 90% at a theoretical protein-loading content of 30 wt %. In vitro protein release experiments revealed a sustained and hyaluronidase (HAase)-dependent release of Herceptin from HMGs, in which 80.6% of Herceptin was released at 1 U/mL HAase in 10 days. The released Herceptin maintained its secondary structure and antitumor activity. In vivo imaging results demonstrated obviously better tumoral retention for Cy5-labeled Herceptin-loaded HMGs following subcutaneous (sc) injection than for the free-protein counterpart. Interestingly, sc injection of the Herceptinloaded HMGs into SKOV-3 human ovarian tumor-bearing nude mice at a dose of 30 mg Herceptin equiv/kg induced nearly complete tumor suppression, which was significantly more effective than the sc or systemic injection of free Herceptin. These tailor-made fluorescent HMGs appeared as a robust injectable platform for sustained and localized delivery of therapeutic proteins.

